Exercise 6: Cyanoacetylene
Lattice Energy Convergence
Initial Analysis
- Open the CAACTY CIF

- Generate Hirshfeld surface
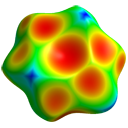 with HF/3-21G electrostatic potential
with HF/3-21G electrostatic potential
Surface Properties
- Analyze electrostatic features:
- Rescale surface to ±0.025 au range
- Note strong dipolar nature:
- HF/3-21G dipole moment: 3.90 D
- Experimental value: 3.72 D
- Observe structured surface features
Deformation Density
- Generate Deformation Density isosurface:
- Use existing wavefunction
- Superimpose transparent Hirshfeld surface
- Compare:
- Electron density accumulation (blue)
- Electronegative surface features (red)
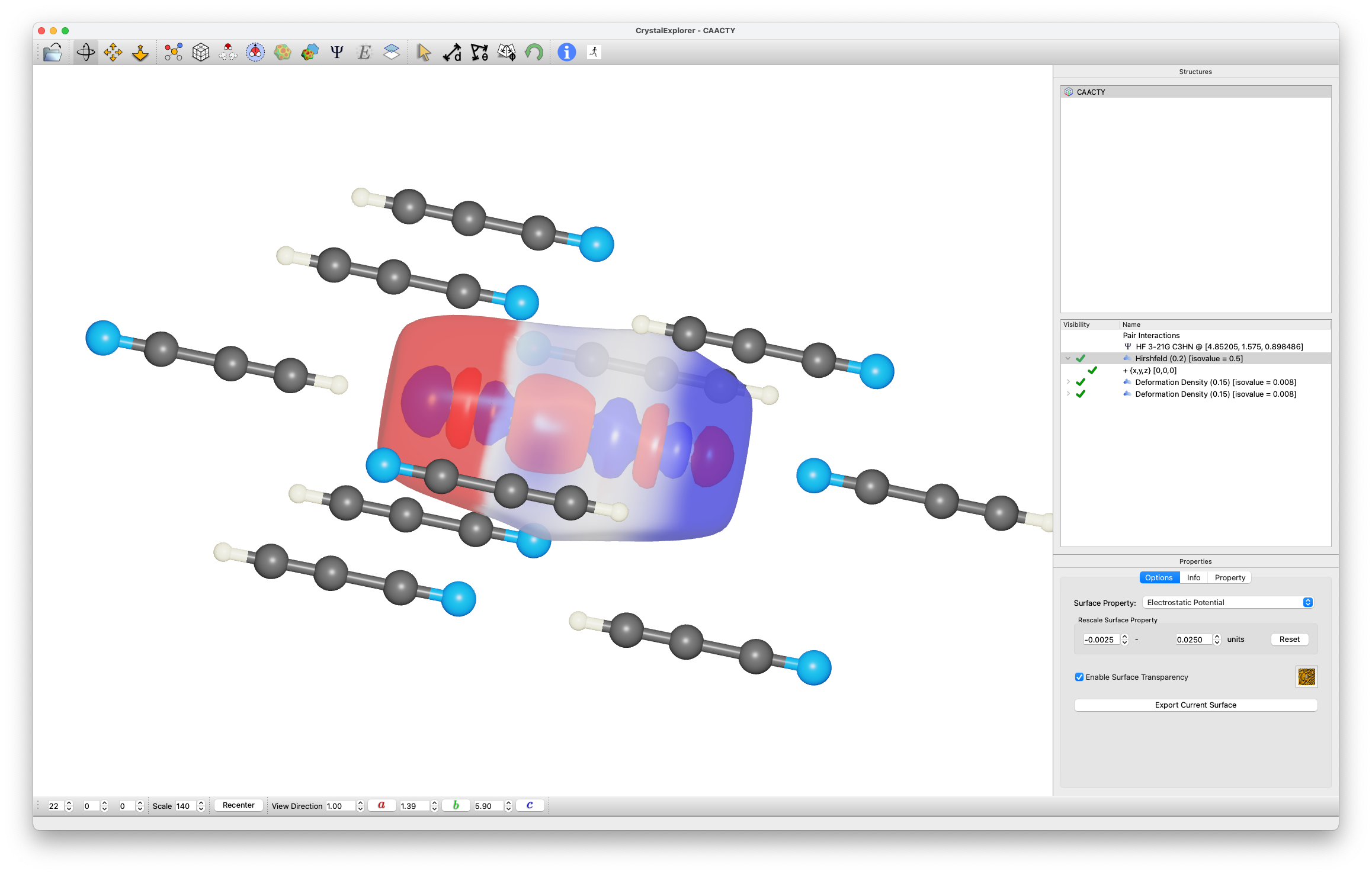
Structure Analysis
- Examine molecular arrangement:
- Use Generate All External Fragments
- Create surfaces with Clone Surface
- Observe structure features:
- Head-to-tail molecular rows in layers
- Alternating layer orientations (antiparallel dipoles)
Historical Context
The original structure determination noted:
- "Linear chains of cyanoacetylene, nearly close-packed"
- "All molecules in a chain oriented alike"
- "Each chain surrounded by two parallel and four antiparallel nearest neighbor chains"
- "No obvious explanation for relative positions of molecules in adjacent chains"
The Hirshfeld surfaces with mapped electrostatic potential help explain these observations
Energy Analysis
- Calculate extensive interaction energies:
- Return to single molecule (Reset Crystal)
- Generate 20 Å radius cluster
- Complete all fragments
- Calculate energies for 230 unique molecular pairs
Data Analysis
Export the energy data to a spreadsheet for detailed analysis:
- Click Information

- Copy energy table
- Paste into spreadsheet
Energy Analysis in Spreadsheet
-
Process the energy data:
- Multiply 'R' and '' columns
- Sort by molecule-molecule distance 'R'
- Observe energy component behavior:
- '' and '': rapid decay
- '': intermediate range
- '': very long range (significant beyond 20 Å)
-
Calculate lattice energy convergence:
- Create partial sum column ''
- Excel formula example:
=0.5*SUM($K$2:K2) - Note convergence around 8 Å
- Plot '' vs 'R':
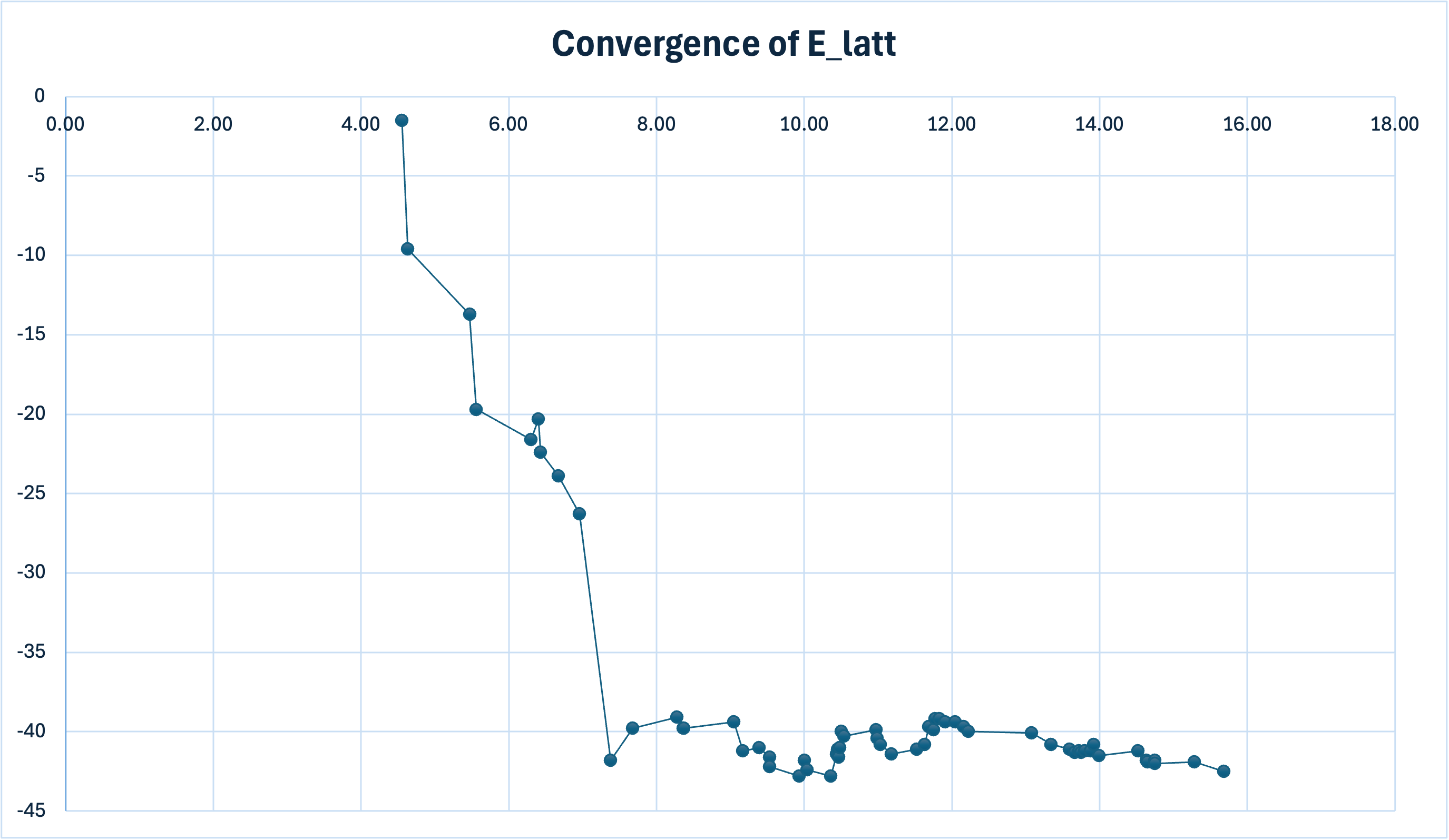
Results
Final lattice energy estimate: -42.3 kJ/mol
Compare with experimental sublimation enthalpy (42.3 kJ/mol)
note
The agreement is fortuitous as these quantities differ by ~2RT (~5 kJ/mol) at room temperature: