Exercise 2: Benzene and Acetylene
Dispersion Interactions Dominate
Initial Setup
- Open the BENZEN CIF
 )
) - Complete the molecule
 (initially shows just unique atoms)
(initially shows just unique atoms) - Generate the Hirshfeld surface (HS)
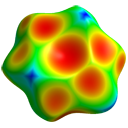 with HF/3-21G electrostatic potential mapped on it
with HF/3-21G electrostatic potential mapped on it
Fingerprint Analysis
- Generate the 'fingerprint plot'

- Note the differences from acetic acid
- Observe the absence of hydrogen bonding 'spikes'
- Look for C--H···π 'wings'
tip
To see the C--H···π feature, filter the plot to show C inside and H outside, including reciprocal contacts
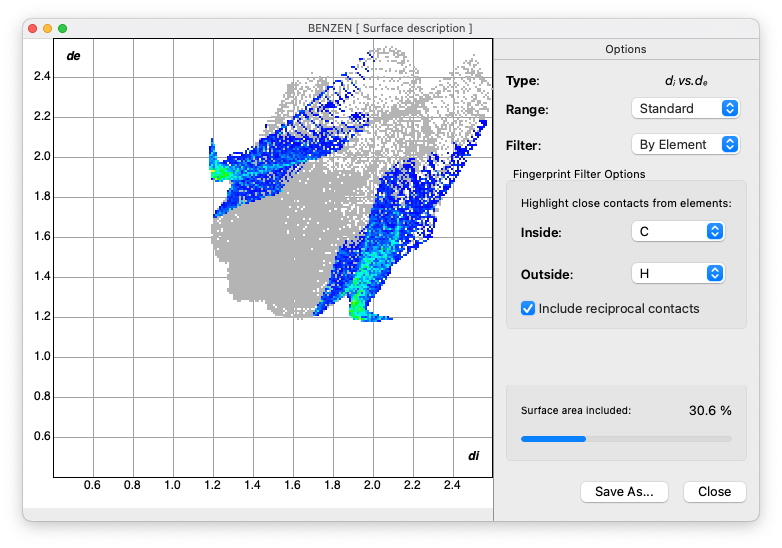
Electrostatic Analysis
- Adjust the electrostatic potential surface:
- Rescale to ±0.025 au range
- Observe:
- Electronegative regions above the ring (carbon π electrons)
- Corresponding electropositive H atoms
Interaction Analysis
- Examine the C--H···π interaction:
- Use Generate External Fragment
- Create molecular pairs using Clone Surface
- Note the electrostatic complementarity between adjacent molecules
Energy Calculations
- Calculate interaction energies:
- Select a molecular pair
- Use Calculate Energies
- Select Energies from user-defined wavefunction
- Expected result: -11.1 kJ/mol
- Note the dominant role of dispersion energy
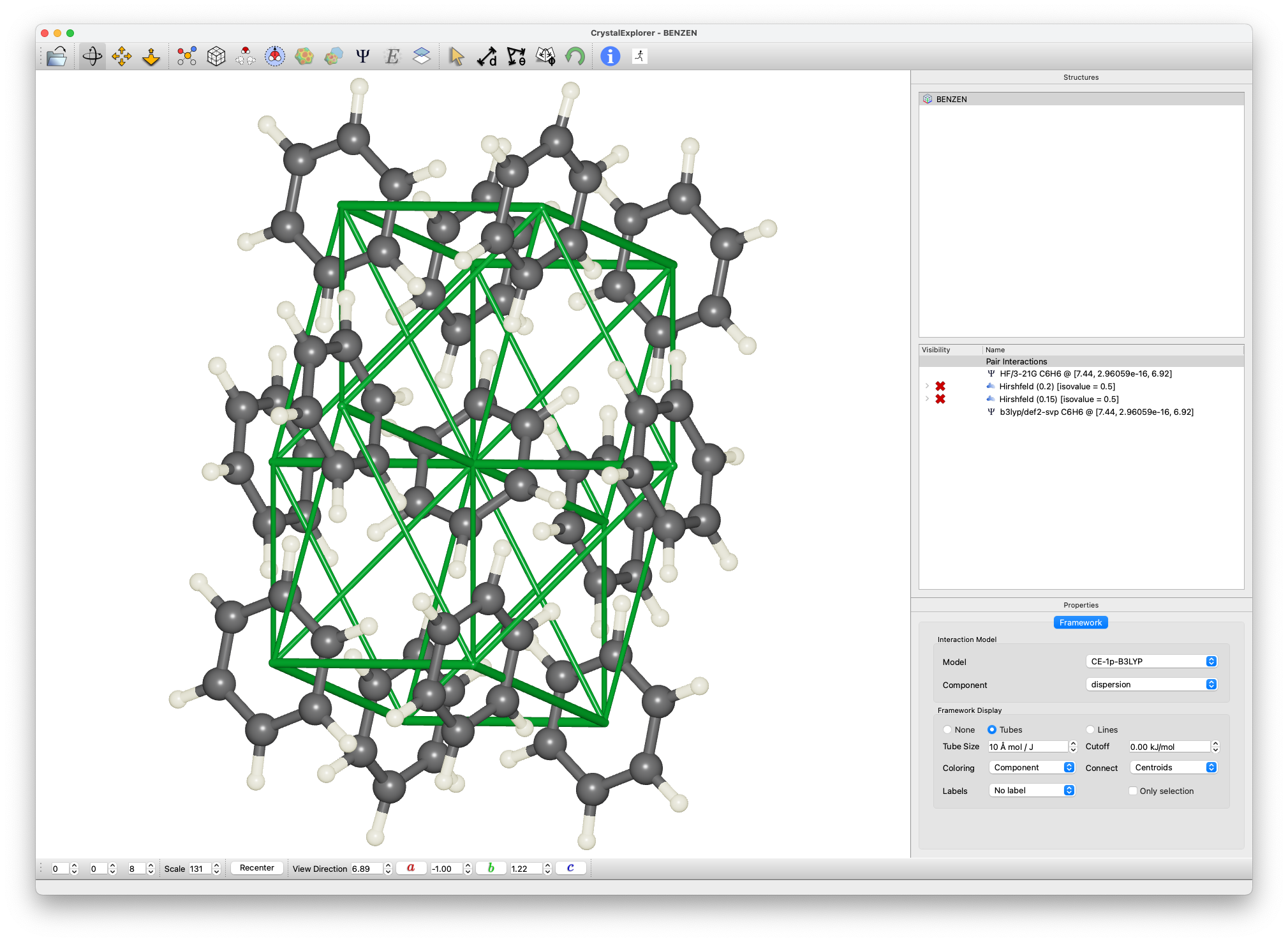
Energy Framework Analysis
- For the cluster analysis:
- Select a molecule and Calculate Energies
- This creates a 3.8 Å cluster
- Computes energies for 3 unique molecular pairs
- Generate energy frameworks:
- Select Display / Energy frameworks
- Compare Coulomb (), Dispersion (), and Total () energies
Lattice Energy
- Calculate lattice energy:
- 3.8 Å cluster result: -47.2 kJ/mol
- 8 Å cluster (15 pairs): -62.4 kJ/mol
- Compare with experimental sublimation enthalpy (~45 kJ/mol)
Acetylene Analysis
Energy Calculations
- Repeat the analysis for ACETYL03:
- All 12 nearest neighbor molecules: -3.3 kJ/mol each
- Lattice energy estimates:
- First shell: -19.8 kJ/mol
- 8 Å cluster: -23.6 kJ/mol
note
This result is close to a converged lattice sum for this non-dipolar molecule