Exercise 4: Hexachlorobenzene
Analysis of a Bending Crystal
Initial Setup
- Open the HCLBNZ11 CIF

- Complete the molecule

- Generate Hirshfeld surface
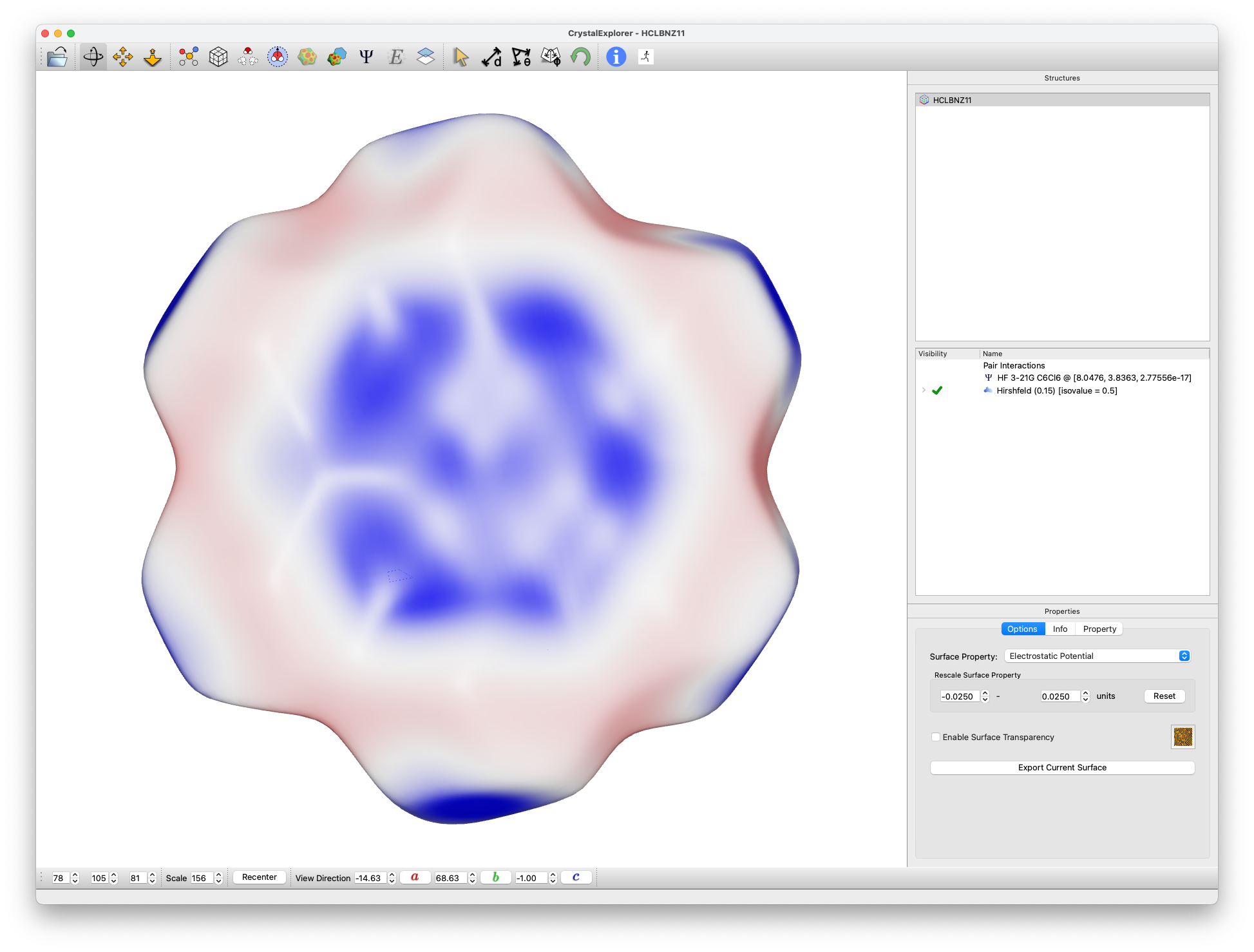
 with HF/3-21G electrostatic potential
with HF/3-21G electrostatic potential
Surface Analysis
- Examine the electrostatic potential:
- Rescale to ±0.025 au range
- Note the smaller range of surface potential
- Observe:
- Electropositive region above ring
- Electropositive regions at C--Cl bond extensions
- Electronegative regions between these areas
Structure Extension
- Build molecular arrangement:
- Create molecules above/below ring using Generate External Fragment
- Generate surfaces using Clone Surface
- Create adjacent molecules using Generate All External Fragments
- Note electrostatic complementarity between molecules
Deformation Density Analysis
- Create Deformation Density isosurface:
- Select Surface Generation / Deformation Density
- Use existing HF/3-21G wavefunction
tip
Choose Resolution / Medium for faster calculation of this structured surface
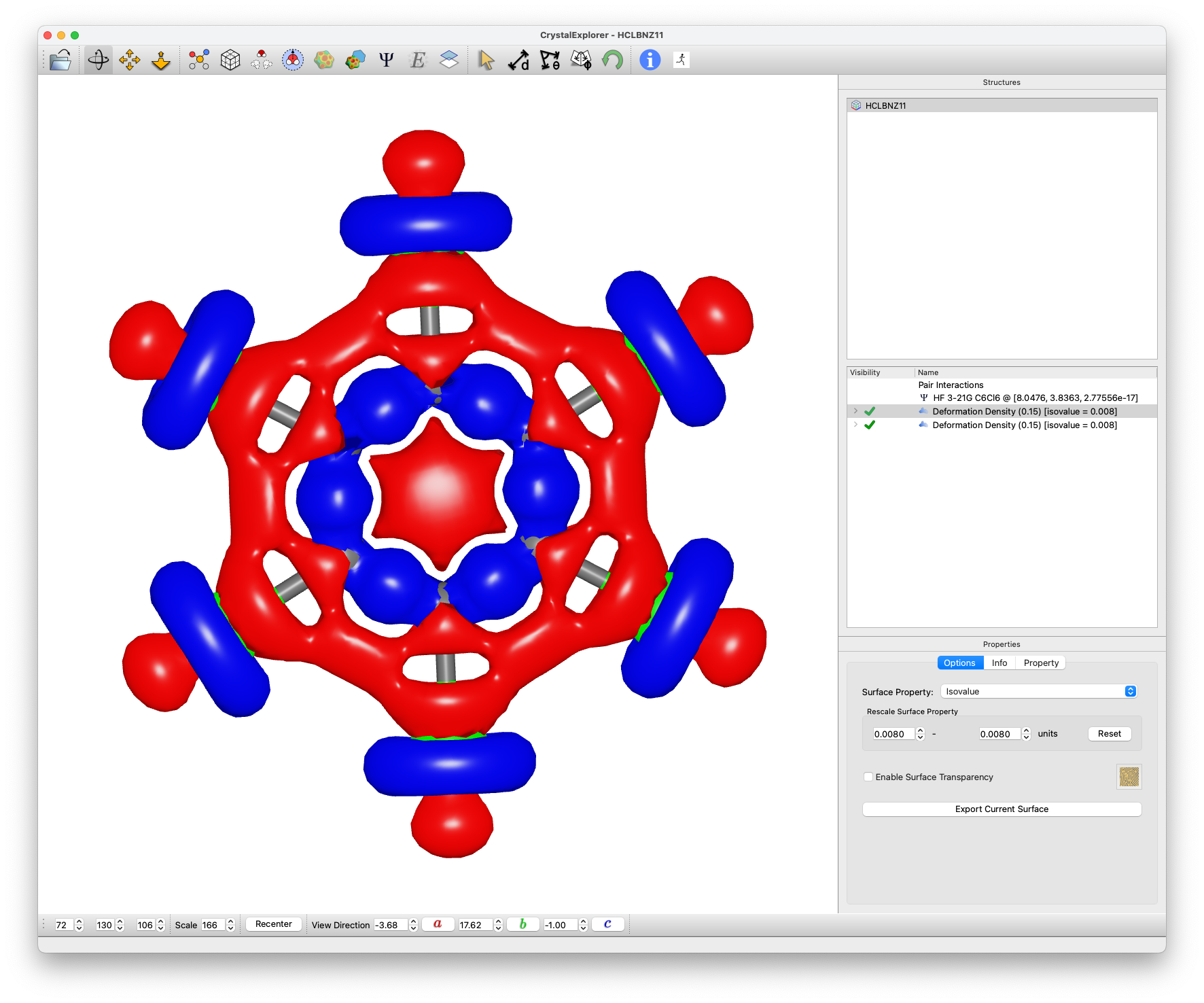
note
Observe how positive electrostatic potential regions at C--Cl bond extensions correspond to electron density deficit areas (red)
Energy Analysis
- Calculate molecular pair energies:
- Return to single molecule (Reset Crystal)
- Calculate energies for 3.8 Å cluster
- Note strongest interaction (-33.0 kJ/mol) along b axis
Energy Framework Analysis
- Generate extended structure:
- Create unit cell molecules (2 cells along a, 3 along b)
- Generate energy frameworks
- Compare components:
- Small electrostatic contribution
- Strong dispersion interactions along b
- Weaker inter-column interactions (also mainly dispersive)
Crystal Bending
This energy distribution helps explain the observed bending of the crystal on the (001) face [C.M. Reddy et al., Angew. Chemie Int. Ed., 12, 2222 (2006)]