Exercise 5: Formaldehyde Acetylene
A Simple Cocrystal
Initial Setup
- Open the GURNEN CIF
 )
) - Complete all molecules

- Generate Hirshfeld surfaces
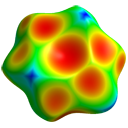 with HF/3-21G electrostatic potential
with HF/3-21G electrostatic potential
Surface Analysis
- Study electrostatic features:
- Rescale surfaces to ±0.025 au range
- Observe:
- Dipolar nature of formaldehyde
- Quadrupolar acetylene surface
Structure Extension
- Build molecular arrangement:
- Create adjacent molecules using Generate External Fragment
- Generate surfaces using Clone Surface
Crystal Engineering
The pattern of electrostatic complementarity supports the original paper's observation that "the packing of formaldehyde with acetylene is such as would be expected by most experts based on crystal engineering principles" [M.T. Kirchner et al., Chem. Eur. J., 16, 2131 (2010)]
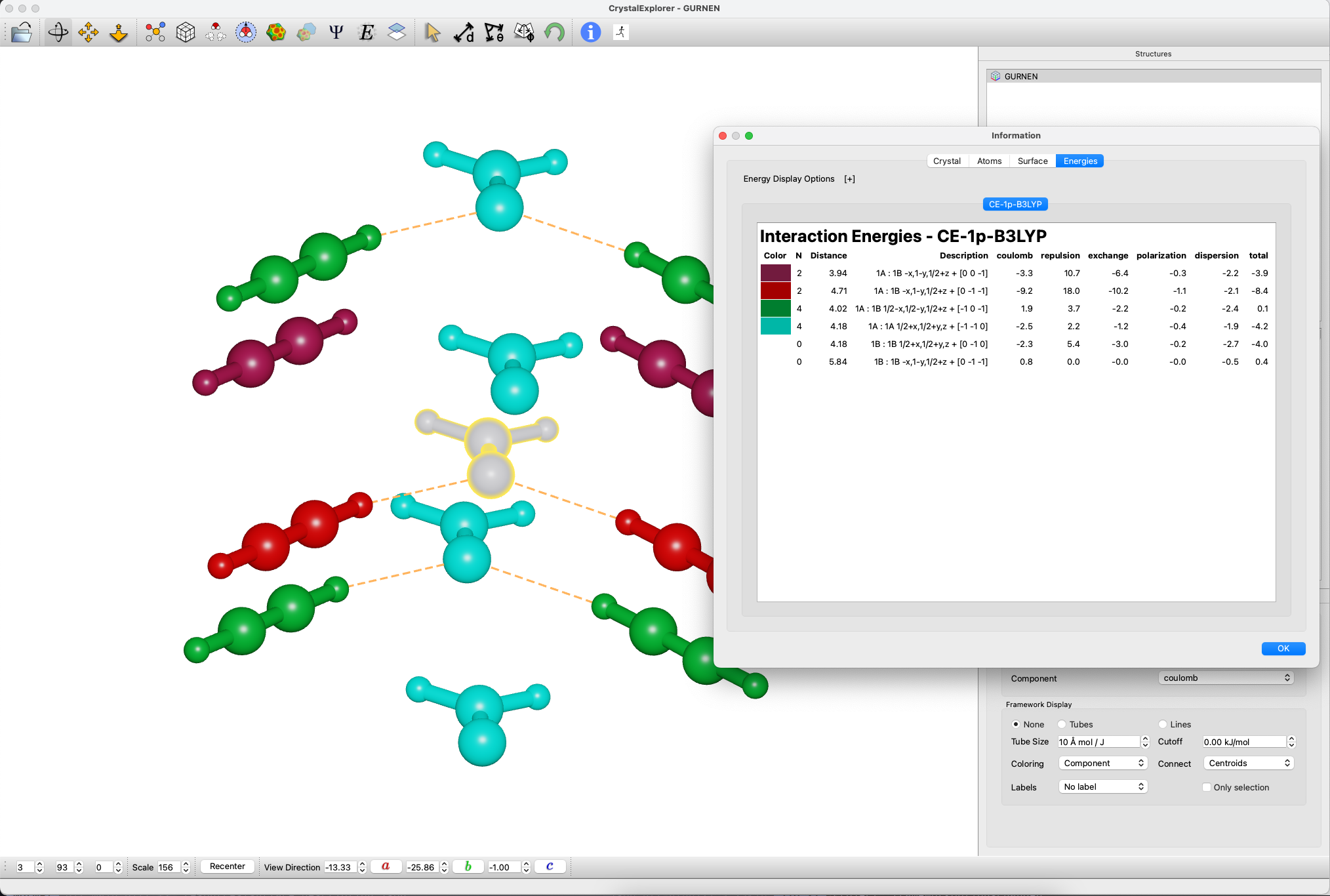
Energy Analysis
- Calculate interaction energies:
- Return to original molecules (Reset Crystal)
- Generate 3.8 Å clusters
- Key interactions:
- C--H···O hydrogen bond: -10.3 kJ/mol
- Formaldehyde dipole-dipole: -4.7 kJ/mol
- Displaced acetylene pairs: -4.7 kJ/mol
Energy Framework Analysis
- Examine energy distribution:
- Generate unit cell molecules

- Create energy frameworks
- Use 4 kJ/mol cutoff
- Observe:
- Zig-zag network of C--H···O interactions in layers
- Weaker interlayer connections
- Generate unit cell molecules