Surface Properties
de and di
is the distance from the Hirshfeld surface to the nearest nucleus outside the surface. is the corresponding distance to the nearest nucleus inside the surface.
and were the original surface properties mapped onto the Hirshfeld surface. Normally, we only map , since this property provides information about the crystal packing environment around the surface.
The simplest and most immediately useful property to map onto the surface is the distance from the surface to the nearest nucleus external to the surface, which we call . This property provides an immediate picture of the nature of intermolecular contacts in the crystal.
In a similar fashion, we can define , the distance from the surface to the nearest nucleus internal to the surface. We have not yet utilised this property as an independent property mapped on the surface, however it is valuable when used in conjunction with .
The range of and across the Hirshfeld surface varies considerably depending on the atoms in the molecule (size dependence) and the particular type of intermolecular interaction experienced (interaction dependence). Mapping de over the same range of all molecules would reduce the colour contrast for molecules which feature only a small range of contact distances (such as hydrocarbons). To gain the maximum benefit from the colour mapping on the surface, we choose a range most suited to the each group of molecules under direct comparison.
dnorm
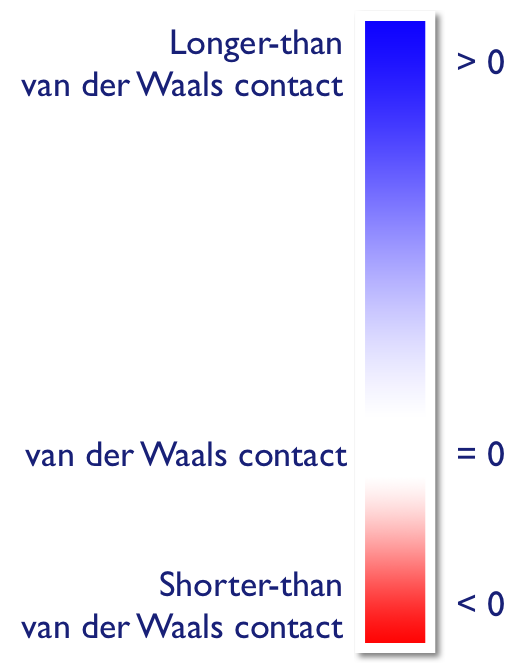
is a normalised contact distance. is normalised by the van der Waals radius of the atom involved; is similarly normalised, and the sum of these two normalised quantities is the property:
where
Where atoms make intermolecular contacts closer than the sum of their van der Waals radii, these contacts will be highlighted in red on the surface. Longer contacts are blue, and contacts around the sum of van der Waals radii are white, as shown in the diagram on the right.
Experimental features
We understand that the van der Waals radius is an arbitrary value for choosing to highlight intermolecular contacts (it is a sensible arbitrary value, though). So, in the case that you might want to highlight contacts that are somewhat greater than the this distance, we have been experimenting with two alternative methods:
- Secondary Highlight: Change the colour scheme so that contacts at the van der Waals separation are yellow, and contacts 5% greater than the van der Waals separation are white.
- Sensitivity:Drag the slider bar to change the white point of the property between 0 and 10% greater than van der Waals separation. This should not be used for publication purposes, as there is no way to reproduce the exact setting you use.
Curvedness and Shape Index
A molecular surface defines the shape of a molecule. Because the Hirshfeld surface defines the shape of the molecule in terms of its surrounding crystalline environment, we think that the local shape of the surface may provide some chemical insight.
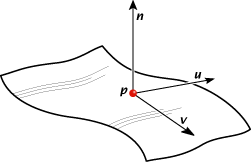
At any point on the surface, we can determine the outward normal, and there exist two principal directions and (see right) along which the principal curvatures and are calculated
The two conventional measures of curvature, the mean curvature, , and the gaussian curvature, , do not provide much physical information. Koenderink12 has introduced two more useful measures of surface curvature, the curvedness, , and the shape index, as follows:
with .
The curvedness is a function of the root-mean-square curvature of the surface, with flat areas of the surface having a low curvedness and areas of sharp curvature having a high curvedness. Areas on the Hirshfeld surface with high curvedness tend to divide the surface into contact patches with each neighbouring molecule, so that the curvedness of the Hirshfeld surface could be used to define a coordination number in the crystal.
The shape index is a qualitative measure of shape and can be sensitive to very subtle changes in surface shape, particularly in regions where the total curvature (or the curvedness) is very low. One important attribute of the shape index is that two shapes where the shape index differs only by a sign represent complementary "stamp" and "mould" pairs. This means that maps of shape index on the Hirshfeld surface can be used to identify complementary hollows (with ) and bumps (with ).