CrystalExplorer
Hirshfeld surfaces, intermolecular interaction energies and more...
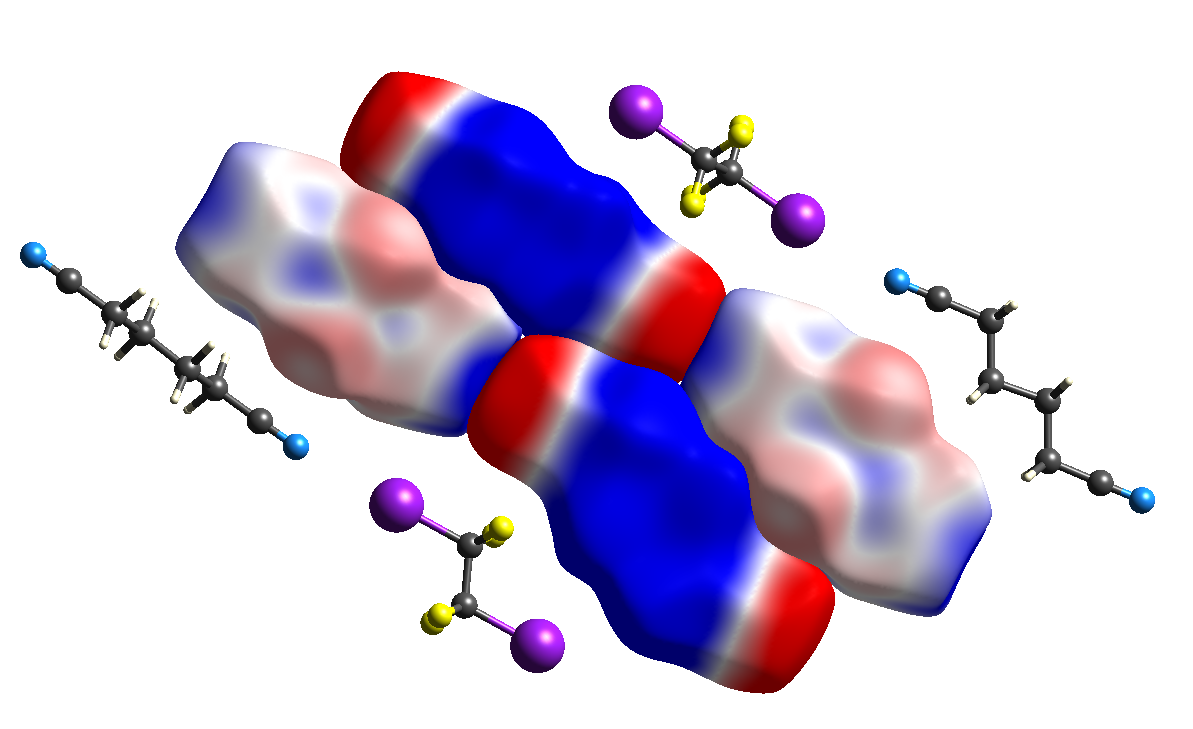
Electrostatic potentials mapped on Hirshfeld surfaces
Mapping the molecular electrostatic potential on Hirshfeld surfaces for a cluster of molecules highlights the electrostatic complementarity between adjacent molecules, providing additional insight into observed crystal packing.
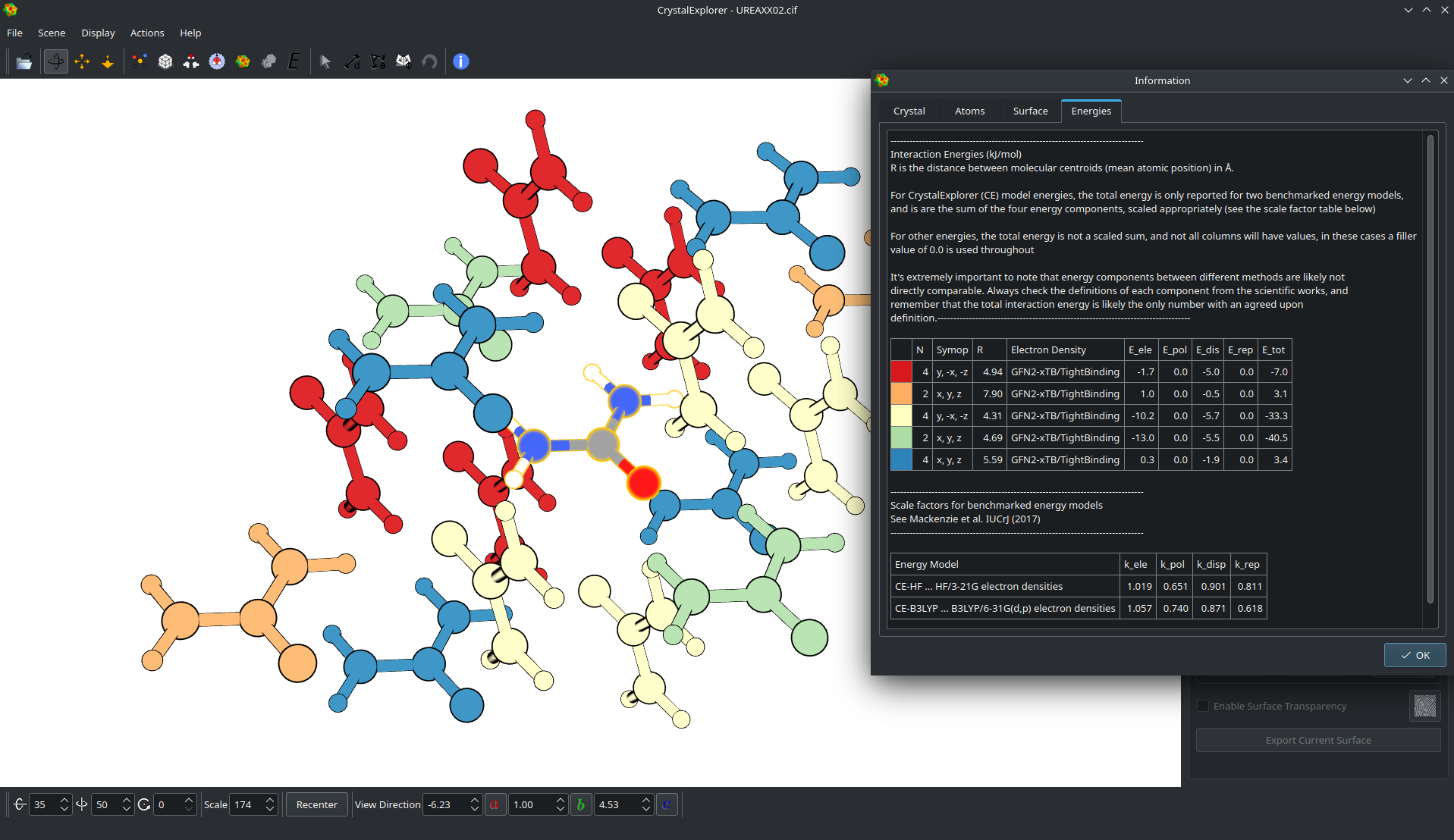
Intermolecular Interaction energies
Intermolecular interactions can be readily quantified using model energies built up from separate electrostatic, dispersion, polarization and exchange - repulsion terms, calibrated against dispersion - corrected density functional theory.
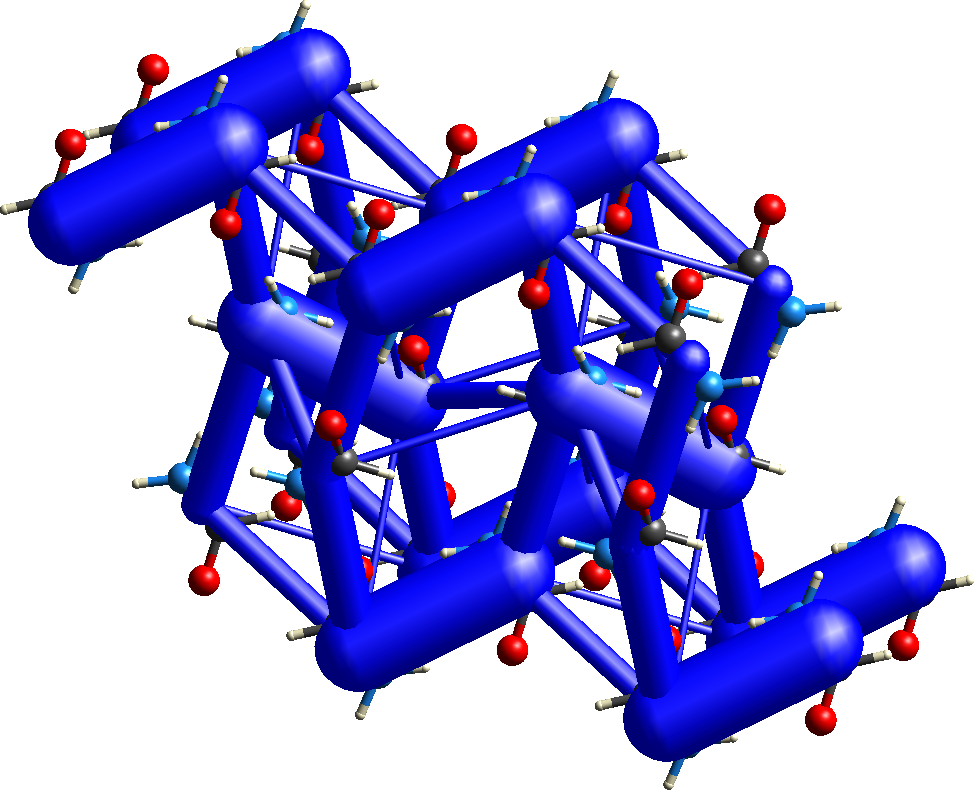
Energy frameworks
The topology of intermolecular interactions in a molecular crystal can be revealed by representing the network of nearest neighbour energies by a framework of cylinders whose width is proportional to the strength of the interaction.Separate frameworks for the electrostatic and dispersion components provide insight into the nature of different interactions.